Technology
Reducing Costs & Improving Outcomes
pCT from Proton Calibration Technologies is an integrated workflow solution that ameliorates a significant issue for proton therapy centers. The clinical need is to efficiently plan and deliver a high-quality treatment safely using large doses and fewer sessions. Join us on our mission to commercialize the world’s fifth medical imaging modality leveraging our precise, proven, proprietary, and profitable pCT technology.
Technology
Proton Computed Tomography (pCT)
pCT is needed due to uncertainty in the range traveled by protons that have passed through various tissues of varying thickness. These variations and the use of X-ray CT to plan treatment give rise to uncertainties in the depth at which the protons will eventually stop. Thus, the beam may deposit its dose partly into a tumor and partly into the nearby healthy tissue. The depth in the body at which the protons stop, i.e., the range, is characterized by the Bragg Peak. The Bragg Peak acts like a scalpel – but we currently only know to a limited extent the boundary at which this scalpel will cut in the patient’s body. Physicians desire precise control over the margins of the excised tumor volume.
The basic problem that gives rise to this challenge is that we do not have an apples-to-apples process. We would like to image, measure, and plan with protons and then treat patients using protons. In current practice, we can only measure with photons (X-rays) and treat patients with protons. Every patient with cancer has an X-ray CT scan. This is used for diagnosis and treatment planning. Unfortunately, since photons and protons behave differently as they pass through the patient, the X-ray CT scan is not sufficient to reduce the range uncertainty. For over two decades, physicians have known the answer is to utilize pCT in the treatment planning process — they just needed someone to invent it.
The calculation starts by considering that each slice contains a number of one millimeter (mm) cubic voxels. For example, a 200mm-by-200mm slice contains 40,000 voxels. We can think of each of these voxels as an unknown variable in a system of algebraic equations. We know that we need at least as many equations as unknowns to solve such a system. We ran the numbers and determined that one can use hundreds of protons, that is equations, for each unknown while limiting the dose absorbed by the patient. In short, with millions of proton trajectory histories there is enough “information”, in the information theory sense of the word, to assure us that pCT is possible.
pCT, at least in its early stages, will use the current generation of proton beam therapy systems to obtain the protons used for imaging. This creates a challenge in that proton therapy systems use a high intensity beam flux so that treatments are administered in a relatively short time. In our first-generation system, we’ll overcome this challenge by placing a robotic aperture between the incoming beam and the patient – the result being a low flux (low intensity) beam suitable for patient imaging. This approach, while practical, is not optimal due to alignment and spatial clearance requirements. In the future, we will work with proton beam therapy vendors to create regulatory-approved low flux mode, thus eliminating the need for the aperture. In succeeding generations, new accelerator systems that will run at higher energies and lower fluxes will be used to obtain superior images at lower patient doses.
It is well known that due to the physics of charged particles, when many protons all enter a patient at the same point and in the same direction, they will exit the patient in multiple directions. It is for this reason that physicists have treated multiple Coulomb scattering as a black box which produces a cone-shaped beam profile. This profile is described by an approximately normal distribution. For our purposes, we are not interested in determining the solid angle of this scattering. We’re interested in the path taken by each individual proton as it transits the patient. Due to the fact that protons are much more massive than the electron clouds with which they interact and the fact that imaging protons exit the patient before exhibiting their Bragg Peak behavior, we can assume that an individual proton takes roughly a straight-line path through the patient. By averaging multiple protons that have passed roughly along the same path, we can correct for the slight deviations caused by multiple Coulomb scattering.

Contact
Proton Calibration Technologies
986 N. Cedar Cove Road
Hartselle, AL 35640
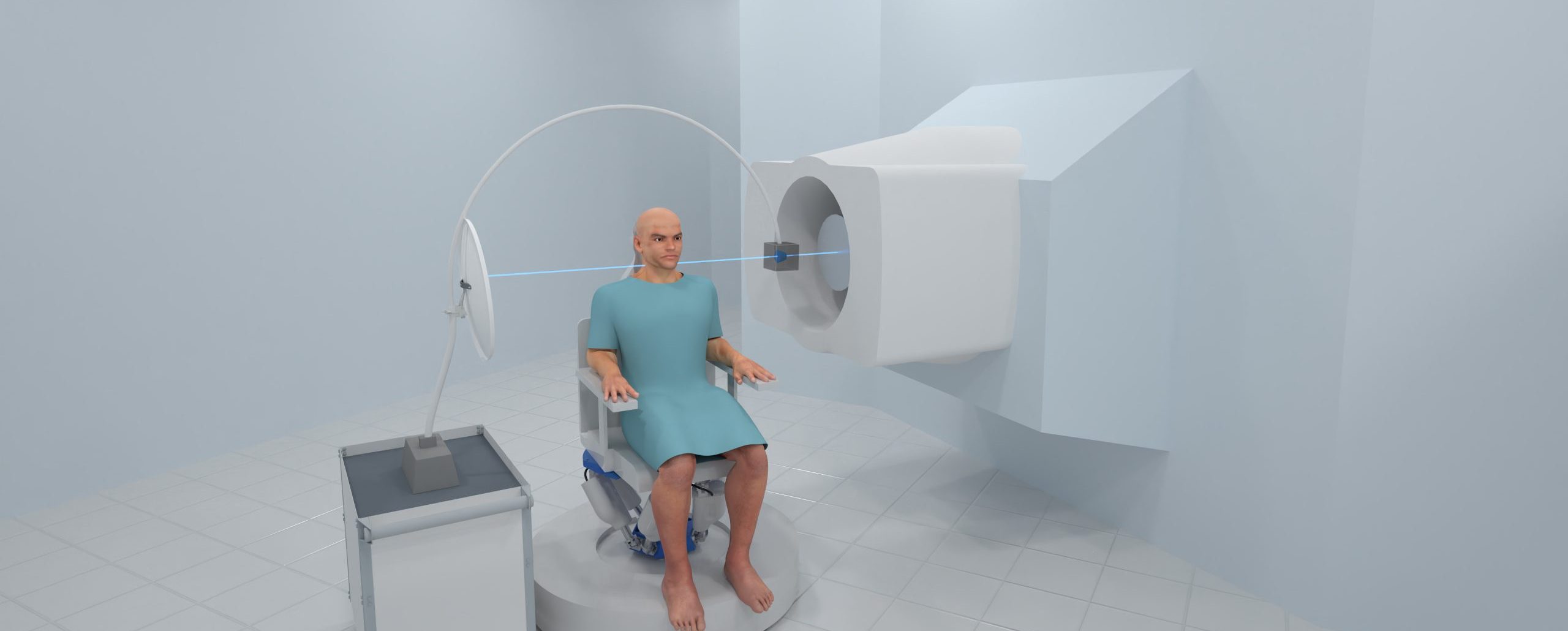
Major Components
First Generation pCT System
A computer controlled alignment system (not shown)
A robotic aperture (the gray box) to decrease the proton flux from therapeutic to imaging levels.
An arm to mechanically and electrically connect the aperture to the remainder of the system
A dish shaped detector to measure the energy of the exiting protons, proton by proton
Before the patient and after the patient location detectors ( not shown) to track the path of the protons
A computer system to support the operator interface, system alignment, patient positioning, beam gating, and image creation.
Boosting Profitability
For Proton Centers
Partnership Opportunities
With PCT
Proton Calibration Technologies is currently accepting applications for the following partnership opportunities. To learn more, contact us today.
Engineering Design
- Systems Engineering
- Systems Integration Design
- Cost of Goods Analysis
- Cost Reduction Analysis
- Medical Device Manufacturing
- Proton Therapy Center Testing

